Abstract
Hydatid disease of the central nervous system is relatively rare and comprises about 2–3% of all the hydatid cyst cases reported in the world. Spinal hydatid disease is an even rarer entity. It is endemic in sheep and cattle-raising regions, seen mainly in Mediterranean countries including Turkey and Syria. Pediatric neurosurgeons in non-endemic countries face a challenge when they encounter children with hydatid cysts of the central nervous system, mostly due to lack of awareness and the ensuing diagnostic dilemmas. It is also a significant socioeconomic problem in developing countries, due to improper hygiene and lack of dedicated veterinary practice. The clinical features are largely nonspecific and very according to location and severity of disease. However, with the advent of advances in MR imaging, the diagnostic accuracy of hydatic disease involving the brain and spine has increased. Intact removal of the cyst/s, without causing any spillage, and appropriate antihelminthic therapy is the goal and key to cure and prevention of recurrence. In this manuscript, the current literature on hydatid cyst of the brain and spine is reviewed to better understand the epidemiology, pathophysiology, diagnostic accuracy, and advances in therapeutic options. A heightened clinical suspicion, awareness of MR imaging features, improved surgical strategies, and options for prevention are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinococcosis is a disease of antiquity, with reports of human affliction by this condition dating back to Hippocrates and Galen, where it was thought to mainly affect the liver of slaughter animals [1, 2]. This disease, however, remains a relevant public health concern in the modern era, with considerable socioeconomic impact on human beings in several parts of the world [3]. The World Health Organization (WHO) has identified echinococcosis as one of the seventeen neglected diseases targeted for control or eradication by 2050 [4].
It is caused by the adult and larval stages of the tapeworm (cestode) belonging to the genus Echinococcus (family Taeniidae). The term Echinococcus was first described by Rudolphi [5]. Cestodes have a predilection for the central nervous system, with hydatid disease of the central nervous system being caused by infestation during the larval stage of the cestode Echinococcus granulosus. Other species, including Echinococcus multilocularis, Echinococcus vogeli, and Echinococcus oligarthrus, have been recognized, but have less relevance in the context of CNS infestation [6, 7]. Parasitic infestation of the central nervous system affects millions of people with a worldwide geographical distribution. A change in the distribution of these parasitic diseases has also become more evident in the era of globalization. Hydatid disease remains endemic in parts of Latin America, Australia, Mediterranean countries, the Middle East, and India, with prevalence rates ranging from 1.2 to 23 per 100,000 inhabitants, especially high where raising sheep, cattle, and other livestock are common practice [8]. The definitive hosts for Echinococcus are canines such as dogs, wolves, and foxes [9].
Hydatid disease of the brain
Pathology and pathophysiology
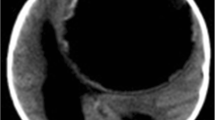
Echinococcus granulosus is the most common genus in humans, who inadvertently serve as intermediate hosts through the accidental ingestion of contaminated food. Viable parasite eggs form oncospheres in the human intestine. These viable Taenia ova later pass through the mucosa of the human intestinal tract into the portal system and spread hematogenously to other organs, mostly the liver, where 75% of the embryos are retained. A further 15% reach the lung and are retained by the pulmonary capillaries, with only 2% of these ova lodging in the brain and 1% in the spine [5, 10, 11]. The hydatid cyst itself has a wall composed of two layers, an inner layer of germinal epithelium (endocyst) and an outer layer laminated hyaline membrane (ectocyst). Hydatid cysts in the brain do not usually have the adventitial membrane (pericyst) surrounding the parasitic organism, which occurs in other parts of the body, unless there has been infection, injury, or rupture of the cyst after which a thick, even calcified adventitial membrane may develop (Fig. 1) [12,13,14]. Daughter cysts and scolices at various stages of development occur in the germinal layer and may detach from the cyst wall and settle at the base of the cyst, forming a sediment. The cyst fluid is generally colorless and contains albumin, glucose, chloride, lymphocytes, scolices, and hooks [5, 6, 8, 15].
Hydatid cysts of the brain are almost always spherical, with a wall that has a whitish to transparent, smooth, thin, slightly elastic consistency, and is made by the parasite and not the host [5]. Growth of the cyst in the brain usually occurs at about 1 cm/year but an increase of 5–10 cm within a year has been reported [16, 17]. While quite rare, hydatid cysts may degenerate and die, typically becoming turbid, with linear calcification, undergoing degeneration and finally shrinkage of the cyst [5, 16].
Cerebral involvement is quite rare, with the majority of cystic hydatid disease of the brain occurring in children, usually transmitted from dog saliva or uncooked contaminated vegetables [18,19,20,21,22]. Cysts are usually single, unilocular, and located supratentorially in the distribution of the middle cerebral artery, but can occur infratentorially [9, 23,24,25,26]. Cyst rupture is uncommon, but can occur spontaneously or secondary to trauma, usually resulting in the development of multiple secondary cysts [23, 25,26,27,28].
Clinical features
Most cerebral hydatid cysts occur in childhood and manifest clinically in early adulthood [29, 30]. The clinical presentation depends on the age of the child, size, number, and location of the cysts as well as the host immune response and temporal evolution of the cyst. Symptoms and signs related to raised intracranial pressure (ICP), i.e., headache, nausea, vomiting, papilledema, impaired level of consciousness, and focal neurological deficit, remain the most common presentation [17, 20, 22, 31, 32]. Seizures remain a less common presentation in this condition, with reports ranging from around 8 to 22% [33,34,35,36,37].
Diagnosis
Serological testing in hydatid disease has limited diagnostic accuracy, with reported sensitivities and specificities of 60–90% [4, 38]. Blood tests should include a C-reactive protein (CRP), estimated sedimentation rate (ESR), and complete blood count which are likely to only show an eosinophilia. The use of antigen tests, ELISA, indirect hemagglutination, and complement fixation tests demonstrate poor diagnostic accuracy, with sensitivities of 25–56% in extra-hepatic disease [4, 38].
Use of enriched antigen and recombinant antigen tests may increase the diagnostic accuracy, demonstrating sensitivities of over 90% on selected serum samples [4, 9, 10, 39]. Protein biomarkers and DNA-based detection methods, such as quantitative or nested PCR assays, provide better diagnostic accuracy. The Casoni skin test has been mostly replaced by serological tests, which are safer and have a better diagnostic accuracy.
Imaging forms the mainstay of diagnosis in patients with a suggestive history and clinical findings, and characteristic imaging findings may still support the diagnosis if serology is negative [9, 10, 39]. CT and MRI form the core imaging modalities for defining the features of cerebral hydatid cysts. Plain CT scan demonstrates a hypodense, cystic lesion with mass effect and midline shift, entrapment hydrocephalus is usually present. There is often not much surrounding edema. Cysts may be a single or multiple in appearance and calcification is thought to suggest previous cyst rupture or infection. Cerebral hydatid cysts appear as large, unilocular, thin-walled cysts, usually without calcification or surrounding edema. They contain fluid with a density similar to CSF on CT scan and MRI. Calcification on CT scan may signify death of the parasite, and irregularity of the cyst wall suggestive of previous rupture [14] (Fig. 2). T1W MRI usually demonstrates hypointense lesion/s, often with minimal surrounding perilesional edema, unless the cyst is infected and eliciting a host inflammatory response. The absence of contrast enhancement and edema are typical of hydatid cyst [40].
T2W MRI usually demonstrates a hyperintense cystic lesion. Intraoperative guidance using ultrasound to identify the cysts and guide the surgical approach and dissection can be a useful adjunct in this context [32, 41].
Management
Medical management
While the mainstay of hydatid disease of the brain involves surgical extirpation, the role of antihelminthic therapy in this context deserves unpacking. Mebendazole was initially used to treat hydatid cysts, but was later replaced by albendazole, which demonstrated improved gut absorption when compared to the former [42,43,44]. The recommended treatment regimens include albendazole at a dose of 12–15 mg/kg/day (given in 2 daily doses, ideally with fatty meals) for 3 months, or at 10–12 mg/kg/day for recurrent 1 month courses, followed by a “rest period” of 15 days after each month [34, 42,43,44]. The optimal duration of treatment is still unclear. Side effects include nausea, vomiting, diarrhea, headache, dizziness, reversible hepatotoxicity, and other gastro-intestinal disturbances, and laboratory monitoring, including liver function tests, should be checked at 2-week intervals for the first 3 months, then monthly is advised [45,46,47].
Praziquantel has demonstrated effectivity as an antihelminthic drug, but does not appear to have a definitive role as a primary therapeutic agent. There is some evidence to suggest that combination therapy with praziquantel and albendazole is more beneficial than albendazole alone [6, 7].
The use of antihelminthic therapy has been recommended in cases of recurrent disease, hydatid dissemination, lesions considered inoperable, or with intraoperative cyst rupture [32]. Use of systemic albendazole before and after surgery has been shown to reduce the recurrence rate [43, 48]. While seizures are rare in cerebral hydatid disease, treatment with appropriate anti-epileptic drugs may be required [32, 48].
Surgical management
Surgery remains the cornerstone of treatment for cerebral hydatid disease, with the goal being intact cyst removal without spillage of the contents. Dowling’s technique remains a popular method of “in toto” cyst extirpation using hydrostatic dissection to define the plane between the cyst wall and brain [49] (Fig. 3). The principles of surgery for intact extirpation of cerebral hydatid cysts mandate an adequate, large craniotomy, meticulous dural opening (especially for superficial cysts), and careful microsurgical dissection of the overlying and surrounding cortex to expose the cyst wall. The finest bore catheters are gently positioned between the cyst wall and the surgical patty-lined underlying cortex (Fig. 4). Normal saline (0.9%) can be used for hydro-dissection of the cyst wall in order to separate it from the brain parenchyma (Fig. 5). Subtle head position adjustment and elevation together with minimal Valsalva by the anesthetist, done timeously, may encourage cyst extirpation (Fig. 6). Hypertonic saline (5%) soaked swabs lining the cyst border are helpful to minimize spillage of the cyst content in cases where there is inadvertent cyst rupture. Always be vigilant to avoid excessive systemic absorption of the hypertonic saline solution. Intraoperative ultrasound may be a useful adjunct in defining the borders of the cyst, and identifying deeply located cysts. Cyst rupture during surgery is almost inevitably associated with recurrence and is an indication for commencing antihelminthic therapy [48]. The consequences of cyst rupture include dissemination, recurrence, and possibly an anaphylactic reaction. Cyst aspiration has been described, but should be considered only when intact removal of the cyst is not possible. Hemostasis, watertight dural closure, and layered tissue and skin closure are essential to limit surgical complications. Usually the brain re-expands to a remarkable extent within months but occasionally postoperative complications such as pneumocephalus, subdural collections or porencephalic cysts, seizures, and transient neurological deficits may occur. Mass effect from the subdural collection or porencephalic cyst may require shunt placement [48,49,50].
Hydatid disease of the spine
Spinal hydatid cysts in children account for 1% of all hydatid disease, and remains a very rare occurrence [51,52,53,54,55,56,57,58,59,60,61,62,63]. There are subsequently very few cases of spinal hydatid disease reported in children and adolescents [61, 62, 64,65,66], which may be partially due to underreporting of this condition, especially in developing countries. Hydatid disease affecting the spine occurs mostly extradurally, often as multiple cysts [55, 62]. Intradural extramedullary cysts are exceedingly rare, with vertebral body involvement occurring in around 0.5–2% of cases [52,53,54,55, 60, 62]. Intramedullary hydatid cysts are the rarest form of this disease, and have been reported in only a few case reports [67,68,69]. Spinal involvement usually results from direct extension from the abdomen, chest cavity, or pelvis, mostly affecting the dorsal area of the spine. The most commonly affected regions of the spine are thoracic (52%), lumbar (37%), cervical (5.5%), and sacral spine (5.5%) [38, 66, 70,71,72]. Spinal hydatid disease can be anatomically and radiologically classified into paraspinal, spinal, and intraspinal, with intraspinal lesions further divided into extradural, intradural extramedullary, or intramedullary (Table 1) [70], or classified according to the route of infection, i.e., primary or secondary disease [38].
Approximately 50% of cases involving the vertebral body also have involvement of the spinal canal, but as the disease spreads under the periosteum and ligaments, the intervertebral discs are spared. The cyst grows slowly, at the rate of 7 mm per month, and results in bone destruction by expansion and mechanical compression, causing ischemia of the nutrient vessels leading to bony necrosis, sequestra formation, and osteoclast proliferation [62]. Intradural extramedullary hydatid disease can present as a giant cystic lesion mimicking an arachnoid cyst [71, 72]. Extradural spread of hydatid cysts through widened neural foramina into the muscle planes may result in a grape-like appearance [54, 58, 66]. Hydatid cyst in the sacral region may mimic an anterior sacral meningocele [73], and purely ventral, extradural hydatid cyst of the spine with no extension into the dura has also been reported [53].
Clinical features
These features can be nonspecific and vary with the location of the cyst. The duration of clinical symptoms can range from acute to prolonged onset. Most commonly, these include back pain, paraparesis, radiculopathy, sensory disturbance, and sphincter involvement. Paraplegia is seen in 26% [55]. Spinal hydatid disease in children, while rarer than adults, has been reported [38, 74,75,76,77].
Imaging
There are no clearly distinguishing radiological findings. Plain X-ray findings are nonspecific, but may show bony destruction, typically of a moth-eaten, osteolytic nature and a soft tissue mass, at single or multiple levels.
CT scan of the spine more efficiently demonstrates the bony erosion and the extent of the lesion. Spinal deformity, if present, is also best demonstrated on CT scan. Calcification demonstrated as a “double layer of arcuate calcification” may be helpful in diagnosing echinococcus [38]. MRI remains the investigation of choice and shows well-circumscribed, cystic lesions, with CSF-like signal intensities, hypointense on T1-weighted imaging, and hyperintense on T2-weighted imaging. T2-weighted images show a low-intensity rim surrounding the homogeneous hyperintense cyst contents (Fig. 7a, b). The cyst wall may be thin and regular, isointense, or demonstrate a slightly lower signal than its contents. A markedly hypointense cyst wall on T1- and T2-weighted MR images is characteristic of hydatid disease. There may be mild enhancement after gadolinium injection, reflecting the vascularity of the pericyst. The differential diagnosis includes arachnoid cyst, arachnoiditis, cystic tumor, tuberculosis, and cysticercosis [62, 72].
Management
Treatment is fundamentally surgical [55, 61, 62, 66]. A laminectomy with total and intact cyst extirpation remains the goal. Precaution must always be taken to avoid spillage of the cyst contents into the intradural or intraspinal space. Hypertonic saline soaked swabs should line the cavity as it destroy the parasites from the cyst fluid. Intact cyst removal remains challenging with vertebral body involvement. When the invasive nature of the infestation in the spine precludes total removal, atraumatic cyst aspiration, especially in multiple extradural cysts, has been described, but remains a secondary option [51, 55, 60, 62]. Correction of spinal deformity in children, where this occurs, usually following multilevel laminectomies, in addition to intact removal of cysts, appropriate anterior, and posterior instrumented stabilization techniques may be indicated [55, 77]. It can often be done in a single setting, preferably under the guidance of intraoperative neuromonitoring. A posterior approach with costotransversectomy is preferred in a kyphotic deformity especially for the resection of the posterior wall of the bony apex [55].
Drug therapy in spinal hydatid disease
Albendazole remains the drug of choice, and the treatment regimens are the same as those for intracranial hydatid disease, as described earlier, where use of albendazole after surgery has been suggested to delay recurrence and reduce complications [51, 59, 61, 62]. Although the reported recurrence rate of spinal hydatid disease ranges between 40 and 90%, patients have an acceptable quality of life following surgery [38, 55, 61, 62, 65]. The risk factors for recurrence include infiltrative and extensive disease, intraoperative cyst rupture, and anatomical location of the disease. Peri-operative use of scolicidal and antihelminthic agents have been advocated to minimize the risk of recurrence in hydatid disease of the CNS [74,75,76].
Advances in management
While most are still in their early stages, there have been several developments in the application of diagnostic technologies which have already improved our understanding of hydatid disease and may be useful for informing new public health strategies aimed at prevention and improved intervention. These developments mostly involve gene transcriptome and proteomic analysis, identification of hormone and cytokine-activated pathways, and improved vaccine development [4].
Conclusion
Hydatid disease should be considered part of the differential diagnosis when dealing with cystic lesions of the brain and spine, especially in endemic regions. Early diagnosis with a high index of suspicion, definitive surgical treatment with intact cyst removal, avoiding intraoperative rupture, followed by a postoperative regimen of appropriate antihelminthic treatment, yields the best outcome.
Availability of data and material
All data and material available as required.
References
Fuchs R (1895) Hippokrates. Sämtliche Werke, München: Lüneburg
Hosemann G (1928) Die Echinokokkenkrankheit. Enke
Eckert J, Thompson RC (2017) Historical aspects of echinococcosis. Adv Parasitol 1(95):1–64
Wen H, Vuitton L, Tuxun T, Li J, Vuitton DA, Zhang W, McManus DP (2019) Echinococcosis: advances in the 21st century. Clin Microbiol Rev 32(2):e00075-e118
Abbasioun K, Amirjam SA (2001) Diagnosis and management of hydatid cyst of the central nervous system. Neurosurgery 11:1–9
McManus DP, Zhang W, Li J, Bartley PB (2003) Echinococcosis. The lancet 362(9392):1295–1304
Moro P, Schantz PM (2009) Echinococcosis: a review. Int J Infect Dis 13(2):125–133
Taratuto AL, Venturiello SM (1997) Echinococcosis. Brain Pathol 7(1):673–679
Eckert J, Deplazes P (2004) Biological, epidemiological and clinical aspects of echinococcosis, a zoonosis of increasing concern. Clin Microbiol Rev 17:107–135
Beggs I (1985) The radiology of hydatid disease. Am J Roentgenol 145:639–648
Tuzun M, Hekimoglu B (1998) Hydatid disease of the CNS: imaging features. Am J Roentgenol 171:1497–1500
Alvarez F, Blazquez MG, Oliver B, Manrique M (1982) Calcified cerebral hydatid cyst. Surg Neurol 17(3):163–164
Obrador S, Urquiza P (1948) Two cases of cerebral abscess of unusual nature: tuberculous abscess and suppurated hydatid cyst. J Neurosurg 5(6):572–576
Peter JC, Domingo Z, Sinclair-Smith C, de Villiers JC (1994) Hydatid infestation of the brain difficulties with computed tomography and surgical treatment. Pediatr Neurosurg 20:78–83
Dew HR (1955) Primary cerebral hydatid disease. Aust N Z J Surg 24(3):161–171
Kemaloglu S, Ozkan U, Bukte Y, Acar M, Ceviz A (2001) Growth rate of cerebral hydatid cyst, with a review of the literature. Child’s Nerv Syst 17:743–745
Sierra J, Oviedo J, Berthier M, Leiguarda R (1985) Growth rate of secondary hydatid cysts of the brain: case report. J Neurosurg 62(5):781–782
Al Zain TJ, Al-Witry SH, Khalil HM et al (2002) Multiple intracranial hydatosis. Acta Neurochir (Wien) 144:1179–1185
Iplikcioglu A, Ozek M, Ozer A, Ozgen T (1992) Periventricular hydatid cyst presenting with hemichorea. Child's Nerv Syst 8:292–293
Fieggen G, Padayachy LC (2015) Chapter 80 - Tuberculosis, parasitic infestation and fungal infections. In: Principals and practice of pediatric neurosurgery, Thieme
Khaldi M, Mohamed S, Kallell J et al (2000) Brain hydatosis: report on 117 cases. Childs Nerv Syst 16:765–769
Padayachy LC, Fieggen AG (2018) Infections in the immunocompromised child. Child’s Nervous System 34(10):1989–1996
Arana-Iñiguez R, San JJ (1955) Hydatid cysts of the brain. J Neurosurg 12(4):323–335
Alok R, Mahmoud J (2020) Successsful surgical treatment of a brain stem hydatid cyst in a child. Case Rep in Surg 2020
Banzo J, Diaz FJ, Pina JI, Abós MD, Rios G, Garcia D, Marin F (1984) Multiple cerebral hydatid cysts. Eur J Nucl Med 9(12):561–563
Rahimizadeh A, Hadadian K (1984) Hydatid cyst of the fourth ventricle. Neurosurgery 14(6):787–788
Abdulla K, Tapoo AK, AGHA HA (1988) Ruptured cerebral hydatid cyst: a case report. J Trop Med Hyg 91(6):302–305
Begg NC, Begg AC, Eobinson EG (1957) Primary hydatid disease of the brain-its diagnosis, radiological investigation, treatment and prevention. N Z Med J 56(312):84–98
Andronikou S, Welman CJ, Kader E (2002) Classic and unusual appearances of hydatid disease in children. Pediatr Radiol 32(11):817–828
Nemati A, Kamgarpour A, Rashid M, Sohrabi NS (2010) Giant cerebral hydatid cyst in a child—a case report and review of literature. BJMP 3(3):a338
Ersahin Y, Mutluer S, Güzelbag E (1993) Intracranial hydatid cysts in children. Neurosurgery 33(2):219–225
Padayachy LC, Dattatraya M (2018) Hydatid disease (Echinococcus) of the central nervous system. Child’s Nervous System 34(10):1967–1971
Altinörs N, Bavbek M, Caner HH, Erdoğan B (2000) Central nervous system hydatidosis in Turkey: a cooperative study and literature survey analysis of 458 cases. J Neurosurg 93(1):1–8
Braunsdorf EW, Schmidt D, Rautenberg M (1988) Cerebral manifestation of hydatid disease in a child. Child’s Nervous System 4(4):249–251
Ciurea AV, Vasilescu G, Nuteanu L, Carp N (1995) Cerebral hydatid cyst in children. Childs Nerv Syst 11(12):679–685
Kocaman S, Ersabin Y, Mutluer S (1999) Cerebral hydatid cysts in children. J Neurosci Nurs 31(5):270
Krajewski R, Stelmasiak Z (1991) Cerebral hydatid cysts in children. Child’s Nervous System 7(3):154–155
Sioutis S, Reppas L, Bekos A, Soulioti E, Saranteas T, Koulalis D, Sapkas G, Mavrogenis AF (2021) Echinococcosis of the spine. EFORT Open Reviews 6(4):288–296
Turgut M (2001) Intracranial hydatosis in Turkey: its clinical presentation, diagnostic studies, surgical management and outcome. A review of 276 cases. Neurosurg Rev 24(4):200–208
Kovoor JM, Thomas RD, Chandrashekhar HS, Jayakumar PN, Pillai S, Shankar SK (2007) Neurohydatidosis. Australas Radiol 51(5):406–411
Padayachy LC, Fieggen G (2014) Intraoperative ultrasound-guidance in neurosurgery. World Neurosurgery 82(3):e409–e411
De Rosa F, Teggi A (1990) Treatment of Echinococcus granulosus hydatid disease with albendazole. Ann Trop Med Parasitol 84(5):467–472
Saimot AG, Cremieux AC, Hay JM, Meulemans A, Giovanangeli MD, Delaitre B, Coulaud JP (1983) Albendazole as a potential treatment for human hydatidosis. The Lancet 322(8351):652–656
Teggi A, Capozzi A, Rosa FD (1989) Treatment of Echinococcus granulosus hydatid disease with mebendazole. J Chemother 1(5):310–317
Brunetti E, Kern P, Vuitton DA (2010) Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 114(1):1–6
Nabarro LE, Amin Z, Chiodini PL (2015) Current management of cystic echinococcosis: a survey of specialist practice. Clin Infect Dis 60(5):721–728
Vuitton DA (2009) Benzimidazoles for the treatment of cystic and alveolar echinococcosis: what is the consensus? Expert Rev Anti Infect Ther 7(2):145–149
Duishanbai S, Dangmuren J, Guo H et al (2010) Intracranial hydatid cysts in children: report of 30 cases. Childs Nerv Syst 26:821–827
Carrea R, Dowling E Jr, Guevera A (1975) Surgical treatment of hydatid cysts of the central nervous system in the pediatric age (Dowling’s technique). Childs Brain 11:4–21
Izci Y, Tüzün Y, Secer HI et al (2008) Cerebral hydatid cysts: technique and pitfalls of surgical management. Neurosurg Focus 24(6):E15
Bhake A, Agrawal A (2010) Hydatid disease of the spine. J Neurosci Rural Prac 1(2):61–62
Dagtekin A, Koseoglu A, Kara E, Karabag H, Avci E, Torun F, Bagdatoglu C (2009) Unusual location of hydatid cysts in pediatric patients. Pediatr Neurosurg 45(5):379–383
Dogan I, Kahilogullari G, Guner E, Unlu A (2015) A rare and unexpected clinical progress and location on a primary extradural spinal hydatid cyst in a pediatric patient: a case report. Childs Nerv Syst 8:1407–1411
Eloqayli H, Matalka I, Daoud S (2010) Primary spinal extradural hydatid cyst in a 4-year-old child. Br J Neurosurg 24(5):602–603
Güneçs M, Akdemir H, Tuğcu B, Günaldi O, Gümüçs E, Akpinar A (2009) Multiple intradural spinal hydatid disease: a case report and review of literature. Spine (Phila Pa 1976) 34(9):E346–E350
Kalkan E, Cengiz SL, Ciçek O, Erdi F, Baysefer A (2007) Primary spinal intradural extramedullary hydatid cyst in a child. J Spinal Cord Med 30(3):297–300
Karadereler S, Orakdögen M, Kiliç K, Ozdogan C (2002) Primary spinal extradural hydatid cyst in a child: case report and review of the literature. Eur Spine J 11(5):500–503
Lakhdar F, Arkha Y, Rifi L, Derraz S, El Ouahabi A, El Khamlichi A (2009) Spinal intradural extramedullary hydatidosis: report of three cases. Neurosurgery 65(2):372–376
Limaiem F, Bellil S, Bellil K, Chelly I, Mekni A, Khaldi M, Haouet S, Zitouna M, Kchir N (2010) Primary hydatidosis of the central nervous system: a retrospective study of 39 Tunisian cases. Clin Neurol Neurosurg 112(1):23–28
Midyat L, Gökçe S, Onder A, Ozdemir Y, Mursalov G, Mir S (2009) A very rare cause of childhood paraparesis: primary intradural extramedullary spinal hydatid cyst. Pediatr Infect Dis J 28(8):754–755
Rumana M, Mahadevan A, Nayil Khurshid M, Kovoor JM, Yasha TC, Santosh V, Indira B, Shankar SK (2006) Cestode parasitic infestation: intracranial and spinal hydatid disease—a clinicopathological study of 29 cases from South India. Clin Neuropathol 25(2):98–104
Singh S, Sardhara J, Singh AK, Srivastava AK, Bhaisora KS, Das KK, Mehrotra A, Sahu RN, Jaiswal AK, Behari S (2016) Spinal intradural hydatid cyst causing arachnoiditis: a rare etiology of cauda equina syndrome. J Craniovertebr Junction Spine 7(4):282–284
Turgut AT, Turgut M (2009) Intradural extramedullary primary hydatid cyst of the spine in a child: a very rare presentation. Eur Spine J 18(8):1234–1235
Bhojraj SY, Shetty NR (1999) Primary hydatid disease of the spine: an unusual cause of progressive paraplegia. Case report and review of the literature. J Neurosurg 91(2 Suppl):216–218
Jaiswal S, Jaiswal AK, Jain M, Behari S, Pandey R (2009) Primary spinal extradural hydatid cyst causing paraplegia. Indian J Pathol Microbiol 52(3):432–433
Sharma A, Kashyap V, Abraham J, Kurian S (1981) Intradural hydatid cysts of the spinal cord. Surg Neurol 16:235–237
Ley A, Marti A (1970) Intramedullary hydatid cyst: case report. J Neurosurg 33(4):457–459
Şenol MG, Tekeli HK, Mustafa Tansel K, Serdar TV, Güner S, Saracoglu M (2012) Intramedullary hydatid cyst of the cervical spine. Indian J Med Microbiol 30(4):480–481
Zhang Z, Fan J, Dang Y, Xu R, Shen C (2017) Primary intramedullary hydatid cyst: a case report and literature review. Eur Spine J 26(1):107–110
Braithwaite PA, Lees RF (1981) Vertebral hydatid disease: radiological assessment. Radiology 140(3):763–766
Rashid M, Kirmani S, Rashid M (2012) Giant intradural extramedullary spinal hydatid cyst—a rare presentation. Clin Imaging 36(6):881–883
Secer HI, Anik I, Celik E, Daneyemez MK, Gonul E (2008) Spinal hydatid cyst mimicking arachnoid cyst on magnetic resonance imaging. J Spinal Cord Med 31(1):106–108
Hemama M, Lasseini A, Rifi L, Boutarbouch M, Derraz S, Ouahabi AE, Khamlichi AE (2011) A sacral hydatid cyst mimicking an anterior sacral meningocele. J Neurosurg Pediatr 8(5):526–529
Ndondo AP, Fieggen G, Wilmshurst J (2003) Hydatid disease of the spine in South African children. J Child Neurol 18(5):343–346
Ozek MM (1994) Complications of central nervous system hydatid disease. Pediatr Neurosurg 20:84–91
Pamir MN, Ozduman K, Elmaci I (2002) Spinal hydatid disease. Spinal Cord 40:153–160
Thaler M, Gabl M, Lechner R, Gstöttner M, Bach CM (2010) Severe kyphoscoliosis after primary Echinococcus granulosus infection of the spine. Eur Spine J 19(9):1415–1422
Author information
Authors and Affiliations
Contributions
LP — writing, editing, and images. MO — editing and images.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed consent was not required as this is a review article.
Consent for publication
No consent required as this is a review article.
Conflict of interest
The authors have no conflict of interest or funding to declare for this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Padayachy, L., Ozek, M. Hydatid disease of the brain and spine. Childs Nerv Syst 39, 751–758 (2023). https://doi.org/10.1007/s00381-022-05770-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-022-05770-7